Research in the Shigeto group uses molecular spectroscopic techniques and data analysis methods including machine learning and multivariate data analysis to study complex molecular systems such as microorganisms. We particularly focus on vibrational spectroscopy (Raman scattering and IR absorption), which provides otherwise unobtainable molecular information. Currently, we are working on the following projects:
- Development of an accurate identification method of microbial species/domains using single-cell Raman spectra and machine learning
- MCR-ALS Raman imaging of microbial cells and their communities
- Multimodal nonlinear microspectroscopy for in situ visualization of plant-mycorrhizal fungal associations
- Raman spectroscopic monitoring of microbial activities under extreme environments (i.e., high pressure and temperature)
- Grain orientation imaging using polarization-resolved low-frequency Raman microspectroscopy
- Raman microspectroscopy of microbial cells cultured using gel-filled microwell array device.
- Study of the formation dynamics of deep eutectic solvents
Among these, three research topics are described in more detail below.
Raman Data and AI
Microbes are essential to the Earth's ecosystem and to our life and health. It is estimated that there are several millions of microbial species living on Earth, but more than 99% of them remain completely unknown. The main reason for this "microbial dark matter" is that microbiology to date has mainly targeted microorganisms that can be easily cultured on agar media and has relied on destructive methods such as genome analysis to analyze the cultured microbes. If we can reveal the species and functions of microorganisms without culturing and destroying cells, it will revolutionize conventional microbiology and have a great impact on various fields such as ecology, medicine, and agriculture.
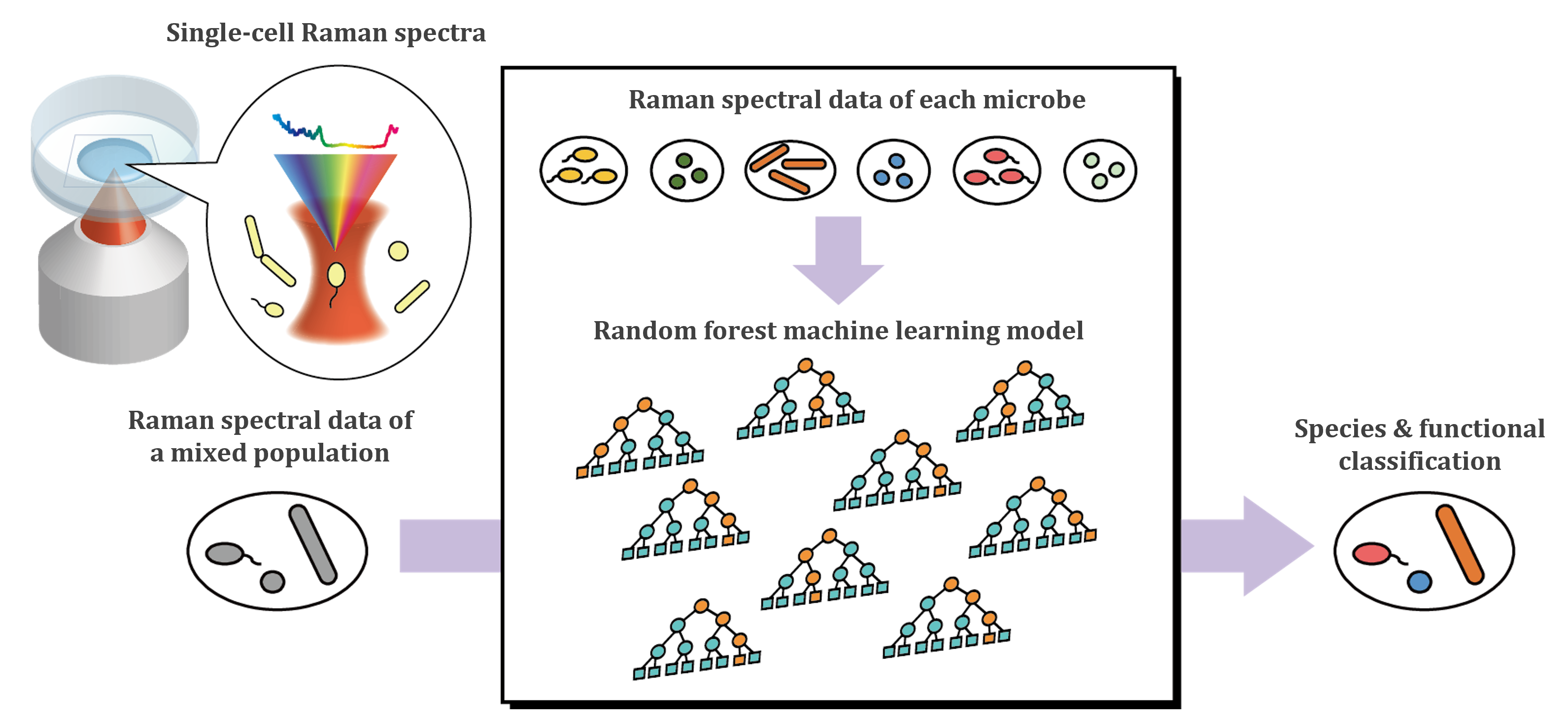
We are developing a method to distinguish microbial species and functions with high accuracy by integrating Raman microspectroscopy, which enables nondestructive analysis of individual cells, with AI techniques (machine learning and deep learning). Using the supervised machine learning algorithms Random Forest and Gradient Boosting, we have successfully discriminated between bacterial and archaeal species based on (often subtle) differences in single-cell Raman spectra [1,2] (Figure 1). We found that the presence/absence of carotenoids, relative amounts of nucleic acids, and differences in the membrane structure are the major contributors to microbial species identification. We also showed that simply combining Raman data from microbial cells in two distinctly different physiological states for model training can significantly improve the discrimination accuracy of microbial species [3].
[1] N. Kanno et al. iScience, 24, 102975 (2021).
[2] N. Kanno et al. STAR Protoc. 3, 101812 (2022).
[3] N. Oda et al. J. Raman Spectrosc. in press (2025)
Linear and Nonlinear Spectroscopic Imaging
Molecular-level understanding of cells, the smallest unit of life, is central to understanding the essence of life. Conventional biochemical methods can only provide averaged molecular information on a large number of cells. Fluorescence-based methods have the drawback that only labeled molecules can be studied. Using Raman microspectroscopy, it is possible to measure in vivo Raman spectra at specific positions within a cell with a submicron spatial resolution. Based on the molecular information obtained from the Raman spectra, we can investigate biological structures and phenomena involving various biomolecules, both known and unknown.
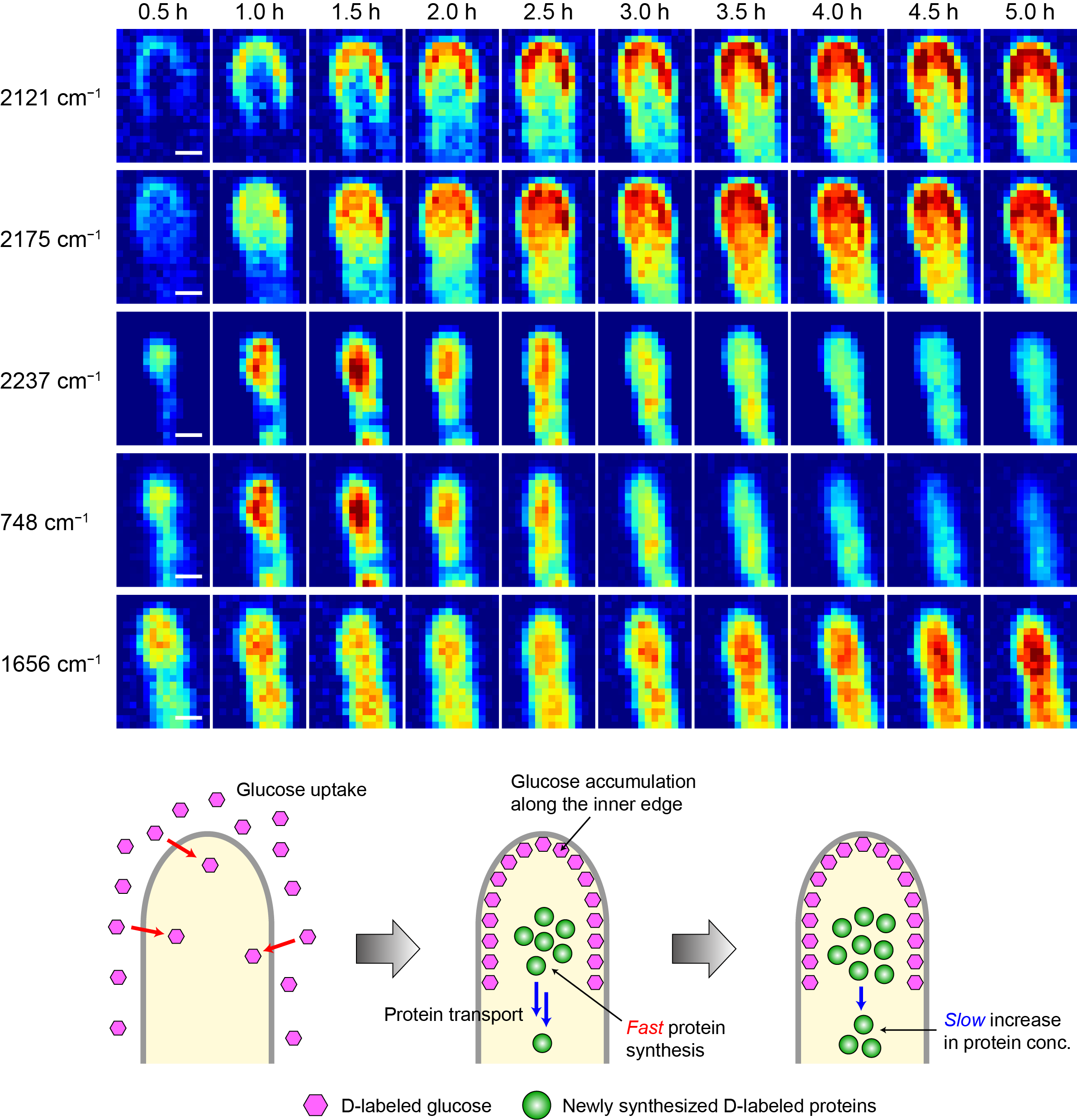
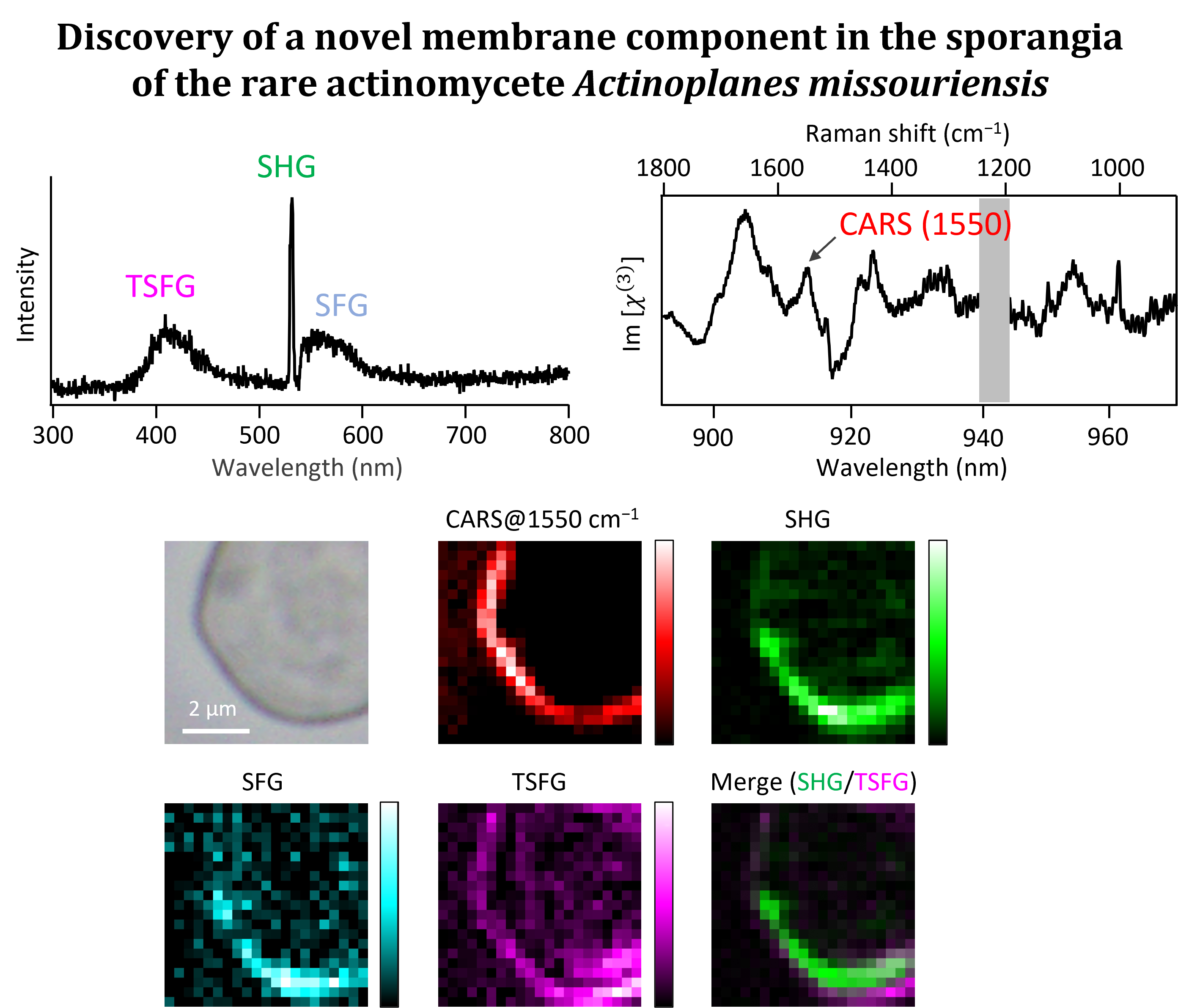
We combine the Raman imaging technique with stable isotope labeling and multivariate curve resolution-alternating least-squares (MCR-ALS) to track uptake and metabolic processes in microbial cells [4] and to visualize the intracellular distribution of molecular components in a label-free manner [5,6]. For example, deuterium-labeled Raman imaging has revealed position-specific uptake and metabolism of glucose into proteins in the hyphal tip of a filamentous fungus (Figure 2). We also use multimodal nonlinear microspectroscopies, which can observe multiple nonlinear optical signals such as coherent anti-Stokes Raman scattering (CARS), second harmonic generation (SHG), and sum-frequency generation (SFG), to simultaneously obtain not only molecular vibrational information but also information on structural order. Recently, we have applied this technique to the unique structure called sporangia produced by rare actinomycetes and discovered a novel membrane component [7]. We have also succeeded in Raman imaging of arbuscules in a plant root that has symbiotic interactions with an arbuscular mycorrhizal fungus.
[4] M. Yasuda, N. Takeshita, S. Shigeto, Sci. Rep. 11, 1279 (2021).
[5] M. Yasuda, N. Takeshita, S. Shigeto, Anal. Chem. 91, 12501 (2019).
[6] R. Sasaki et al. J. Phys. Chem. B 127, 2708 (2023).
[7] K. Usami, T. Tezuka, Y. Ohnishi, S. Shigeto, ACS Omega, 9, 39956 (2024).
Low-Frequency Raman Spectroscopy
Raman spectra in the low-frequency region, which are very close to Rayleigh scattering, show Raman bands originating from intermolecular vibrations and crystal lattice vibrations (phonons), unlike those in the fingerprint region. The former provides information on intermolecular interactions such as hydrogen bonding, whereas the latter sensitively reflects the crystal structure. In addition, the intensity ratio of both Stokes and anti-Stokes Raman scattering signals observed in the low-frequency region can be used to directly determine the temperature of molecules in a sample [8]. Recently, a special optical component called a volume Bragg grating has been developed and can be used as a notch filter to easily measure Raman scattered light at extremely low frequencies of less than ±10 cm-1.
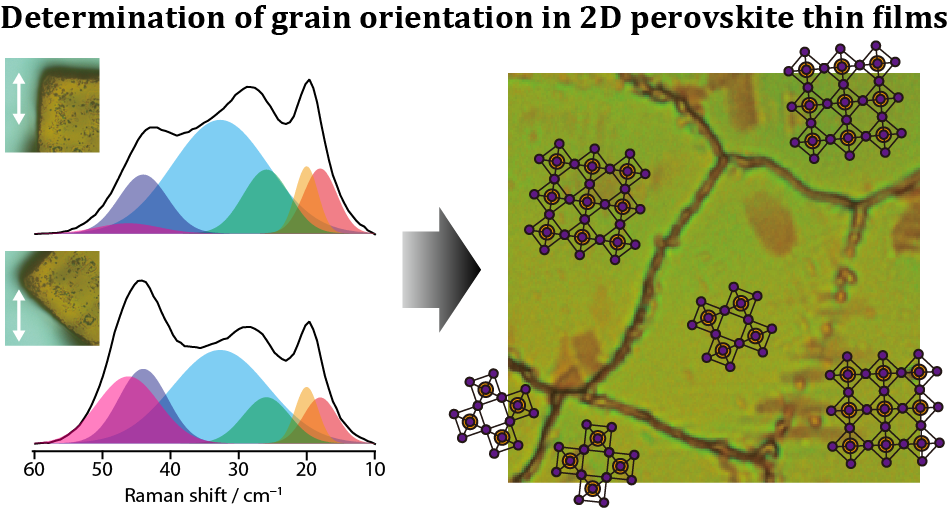
We have developed a method to visualize the orientation of grains in thin films at atmospheric pressure using polarization-resolved low-frequency Raman microspectroscopy [9,10]. We applied this method to thin films of CH3(CH2)3NH3PbI4, a prototype of two-dimensional (2D) organic-inorganic hybrid perovskites that have attracted much attention as light-absorbing materials for next-generation solar cells, and determined the absolute orientation of the grains (Figure 4). It is expected to be applicable to orientation imaging of thin films consisting of microcrystals stacked parallel to the substrate, such as this 2D perovskite.
[8] Y. Yoshikawa, S. Shigeto, Appl. Spectrosc. 74, 1295 (2020).
[9] S. Toda et al. J. Phys. Chem. Lett. 11, 3871 (2020).
[10] S. Toda, E. W.-G. Diau, S. Shigeto, J. Phys. Chem. C 125, 27996 (2021).