Research in the Shigeto group utilizes physicochemical approaches based mainly on vibrational spectroscopy (Raman scattering and IR absorption), which provides otherwise unobtainable molecular information. Currently, we are working on the following projects:
- Interaction and heterogeneity in microbial communities
- Development and application of a "Raman cell thermometer"
- Intermolecular interactions and formation mechanism of amyloid fibrils
- Hydrogen bond structures of nanoconfined water
- External electric field effects on organic–inorganic hybrid perovskites
In what follows, some of our ongoing as well as previous studies are outlined.
Microbial Community
We cannot live without microorganisms. For instance, gut flora profoundly affects our health; most of fermented foods such as cheese, yogurt, and natto are produced with the help of bacteria or yeasts; and bioremediation including wastewater treatment takes advantage of microorganisms. On the other hand, microorganisms also represent a large threat to us, as in the case of bacterial infections. Understanding and controlling microbial functions will therefore have tremendous impact on our society.
Since more than 90% of microorganisms that exist on the earth are thought to spend their lives in communities rather than in solitude (i.e. in a free floating state), it is crucially important to study such microbial communities. We analyze biofilms, structured communities of bacterial cells formed on surfaces/interfaces, at the molecular level and in a nondestructive manner. Microorganisms we are studying include Lactobacillus plantarum, Paracoccus denitrificans, and a bacterium that corrodes stainless steel. We use the stable isotope-labeled Raman microspectroscopy and imaging that we have developed and tip-enhanced Raman scattering (TERS) to reveal the interactions and heterogeneity in microbial communities in terms of the spatial distributions and formation rates of various metabolites. Our ultimate goal is to elucidate how microorganisms, which we are apt to consider lower organisms, form their "society" for survival.
Fig. 1: An illustration of a biofilm. Slimy stuff in a sink or in a bathroom is a familiar example of biofilms.
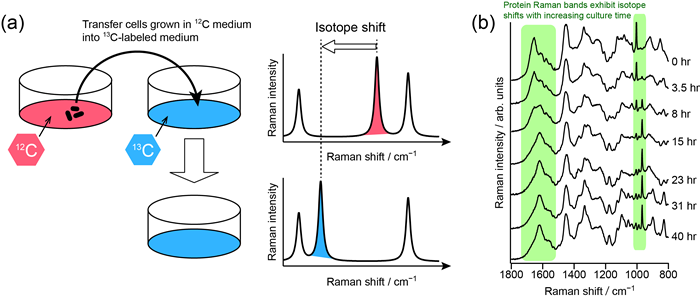
Fig. 2: (a) Principle of stable isotope (13C) labeled Raman spectroscopy. (b) Application to fission yeast cells.
[H. N. Noothalapati Venkata, S. Shigeto, Chem. Biol. 19, 1373 (2012)]
Single Living Cells
Cells are the smallest unit of life, and hence molecular-level investigation of cells provides key insight into what is life. Conventional biochemical methods only provide molecular information that is averaged over a number of cells. Fluorescence microscopy, which is now widely used as a powerful tool in biological studies, is in principle limited to probe target molecules that are fluorescently labeled. In contrast, Raman spectroscopy can look at living cells as they are, with submicron spatial resolution. Detailed analysis of space-resolved Raman spectra obtained from a cell enables us to investigate dynamic cellular processes like cell division and cellular metabolism with a view to understand, e.g., how cells become cancerous or aged. To overcome the difficulty arising from the fact that Raman bands of many different biological molecules overlap in cellular Raman spectra, we employ alternating least-squares–multivariate curve resolution (ALS–MCR). We have demonstrated that ALS–MCR successfully disentangles the Raman spectra of individual components such as lipids and proteins and visualizes their intracellular distributions. We are also developing a novel method using Raman spectra for measuring intracellular temperature without the need of introducing temperature sensors into a cell.
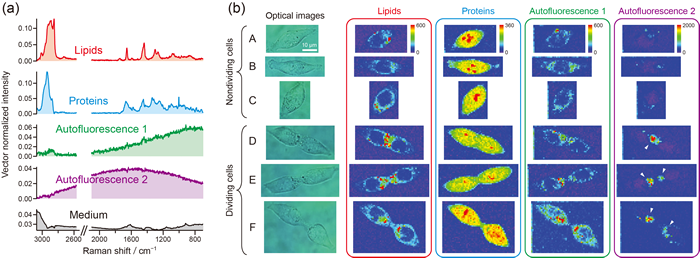
Fig. 3: ALS–MCR Raman imaging of human colon cancer cells (HCT116). The Raman spectra of lipids, proteins, and autofluorescence components have been clearly resolved.
[J.-F. Hsu, P.-Y. Hsieh, H.-Y. Hsu, S. Shigeto, Sci. Rep. 5, 17541 (2015)]
Electric Field Effects on Molecules
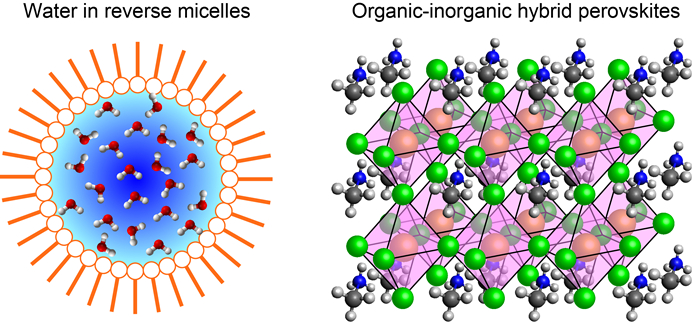
A molecule responds to an external electric field in many different ways. The electronic properties of the molecular would be changed. If the molecule is polar, it would undergo reorientation along the applied electric field. Furthermore, chemical equilibria might be affected by the electric field as well. We use an IR electroabsorption spectrometer that is unique to us to detect such molecular responses as changes in IR absorbance, ΔA, with an unprecedented sensitivity of ΔA ≈ 10−8. We are currently studying water molecules that are confined on a nanometer length scale to better undertstand the hydrogen bond structure of water in nanoconfinement. Water in reverse micelles and water dissolved in 1,4-dioxane are used as model systems for confined water. In addition, we have recently started applying our method to observe the electric field effect on the organic cation in the organic–inorganic hybrid perovskite CH3NH3PbX3 (X = Cl, Br, and/or I) thin films. CH3NH3PbX3 has been attracting keen interest because solar cells fabricated using this perovskite semiconductor as the light absorber are shown to achieve a power conversion efficiency (PCE) exceeding 20%. Despite intensive studies, the role of the organic cation CH3NH3+ in the PCE and stability of perovskite solar cells still remains elusive. We are trying to shed new light on the role of CH3NH3+ by observing and analyzing the electric field effect on CH3NH3+ IR bands.
Fig. 4: Primary targets of our IR electroabsorption spectroscopic studies.