|
Epithelial
cells perform their physiological functions by organizing into
three-dimensional tissue structures. One of our laboratory focuses is on a
protein called epimorphin (Hirai et al., Cell, 1992), that we have shown to
stimulate epithelial cells to organize into three-dimensional structures and
undergo functional differentiation in vitro. Although epimorphin lacks a signal
peptide for secretion and has a transmembrane domain at the C-terminus, this
protein is released
from the stromal fibroblast and acts as a morphoregulatory protein for
epithelial cells. By understanding the molecular mechanisms underlying
epimorphin's action this may establish a novel concept regarding stromal influence
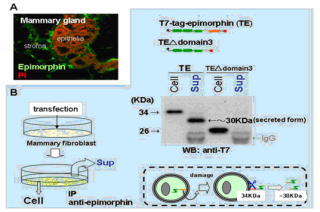
on epithelial behaviors. This year, we addressed how epimorphin is presented at
the outer cell surface without a signal peptide, secreted to pass through the
basement membrane and captured by the target epithelia. We found that 1)
cellular damage leads to extracellular projection of epimorphin, 2)
extracellular epimorphin is cleaved near the C-terminal hydrophobic domain to
be secreted, and 3) alphaV- containing integrin on the target epithelia
binds to this secreted epimorphin. Given that active tissue morphogenesis
includes the process of apoptosis in certain cellular populations, cellular
damage-dependent epimorphin release could be an important mechanism allowing
epimorphin's extracellular action, albeit other mechanisms may also be exist.
By combination of epimorphin with other molecules for morphogenesis we will try
to establish technologies to control the morphological differentiation of
various tissues. The target cells mainly used in the lab will include primate
ES cells and its epithelial derivatives. (By Hirai, Y.)
Another project we are working on is the analysis of tight junction formation
of keratinocyte. The wound healing is one of the regeneration systems that are
intensively studied on. During this process, cells surrounding the wound move
directionally toward this wound. For this directional moving, cell-cell and/or
cell-ECM interaction are regulated cooperatively. Recent study demonstrated
that cell-ECM interaction is regulated by the JNK, c-Jun N-term kinase.
Meanwhile regulatory mechanism of cell-cell interaction during this process is
still unclear. Recently, we found that the localization of ZO-1, one of the
components of tight junction, change dramatically in HaCaT, human epidermal
keratinocyte cell line, when this cell line is cultured with JNK inhibitor.
This result suggests that the tight junction formation of HaCaT is repressed by
JNK activity. Now we are analyzing the molecular mechanisms underlying this
process for expecting that this study uncover the regulatory mechanism of
cell-cell interaction during the wound healing. (By
Aono, S.)
|





