|
Epithelial cells perform their physiological
functions by organizing into three-dimensional tissue structures. One of our
laboratory focuses is on a protein called epimorphin, the temporal
extracellular projection of which has been shown to control the overall
structure of tissue architectures. Epimorphin lacks a signal peptide for
secretion and the major population remains in the cytoplasmic surface of the
membrane, where it functions as a membrane fusion mediator t-SNARE protein.
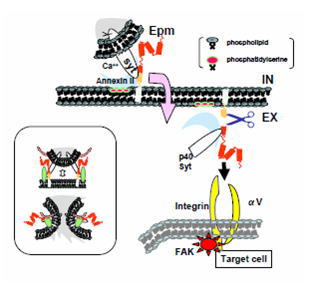
Then, how this molecule translocates across the membrane and gets accessible to
the target cells to elicit their morphogenic responses? By the detailed
analyses of epitope-tagged epimorphin and its related family members, we found
this year that 1) cellular damage or calcium influx leads to extracellular
projection of a secretory complex containing epimorphin, annexin II and
extravesicular domain of synaptotagmin, 2) extracellularly presented epimorphin
is specifically cleaved between E245 and H246 in the SNARE domain and released
toward the target cells, and 3) secreted epimorphin is captured by integrin
cell surface receptors to activate focal adhesion kinase in the target cells.
These results suggest a new model for consequence of epimorphin's extracellular
action (Fig. 1). We are now trying to establish the functional relationship
between epimorphin's intracellular membrane fusion function and extracellular
morphoregulatory function (that apparently distinct roles are encoded in a
single molecule should have a biological significance). Also, we will analyze
the effect of extracellularly presented epimorphin on the multicellular
arrangement and functional differentiation of keratinocyte in 3-D cultures
using HaCaT model. (by Hirai, Y.)
Another research project currently working on is
the analysis of regulatory mechanisms of tight junction (TJ) formation in
keratinocyte. Recently, we found that the localization of ZO-1, one of the
components of TJ, changes dramatically in HaCaT, human epidermal keratinocyte
cell line, when this cell line is cultured with JNK inhibitor. Moreover
claudin-4, another components of TJ, was newly phosphorylated during this
process. To understand the biological significance of this claudin-4
phosphorylation, mutant claudin-4 proteins in which putative phosphorylated
amino acid was substituted to alanine were introduced into HaCaT and examined
the effects on TJ formation. Then, one of mutant claudin-4 molecule S195A in
which 195th serine is substituted to alanine, showed dominant-negative effect
on TJ formation. These results strongly suggest that TJ formation of HaCaT is
regulated by the phosphorylation of claudin-4. Now, we are looking for the
kinase which phosphorylates 195th serine of claudin-4 in HaCaT. (by Aono, S)
|





