
|
| INTORODUCTION |
| 2007 |
2006 |
2005 |
2004 |
Department of morphoregulation
was established in October 2004 by a donation from Sumitomo Electric
Industries, LTD. Our laboratory focus is on a protein called epimorphin (Hirai
et al., Cell, 1992) that we have shown to stimulate epithelial cells to
organize into three-dimensional structures and undergo functional
differentiation in vitro.
Epithelial cells (including hair follicle epithelium) perform their
physiological functions by organizing into three-dimensional tissue structures.
Our research has attempted to use the same strategies used by differentiated
hair cells to design a therapy to stimulate hair growth. Cells organize into
functional tissues by establishing proper cellular polarity, increasing
cell-cell contacts, and by secreting signaling molecules that enable cellular
cross-talk. We have found that the epimorphin protein is capable of stimulating
tissue organization by affecting these specific cellular properties. By the use
of three-dimensional cell culture assays we have been able to study the effects
of epimorphin on hair follicles in vitro.
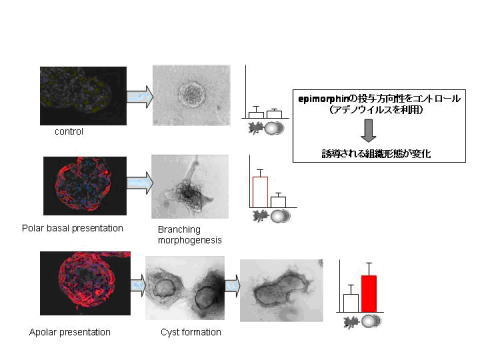
Our lab discovered the mesenchymal protein epimorphin in 1992. Since our
initial discovery Epimorphin has been shown to stimulate epithelial
morphogenesis in a wide variety of organs, albeit the mechanism of its cell
surface presentation is still unclear. For example, experiments both in culture
and in transgenic mice demonstrated that this protein is necessary for mammary
epithelial cells to undergo normal morphogenesis. We have also shown that
epimorphin can stimulate different types of morphogenesis when presented to
mammary epithelial cells using different approaches. Interestingly, the
epimorphin protein gives rise to insoluble oligomeric products through intra-
and inter molecular interactions, and it is thought that this process allows
for epimorphin to directly bind to the surface of target epithelia. In
addition, a number of molecules that signal downstream of epimorphin have been
identified by our lab and others. Our lab has developed a virus system in order
to overexpress exogenous extracellular epimorphin and by using RNA interference
(RNAi) we have inhibited endogenous epimorphin. Also notable, the recent
progress from this research has made possible a hair-growth reagent derived
from epimorphin. This therapy was developed successfully by identifying the
cellular recognition domain from epimorphin that is responsible for stimulating
hair growth. By genetically engineering amino acid mutations in the isolated
sequence the potency of the molecule has been optimized. The generated peptide
has a strong affinity to hair follicles stimulating a turnover from telogen to
anagen phase and it necessitates a very low concentration (only a 1/10000
dilution of epimorphin is required to see the same effect an active component
from a conventional hair growth tonic can cause). This concentration is almost
identical to the endogenous concentration of other growth factors for signaling
to target cells, suggesting that the small molecule derived from the epimorphin
protein, at least for hair follicle regeneration, is now in our hands. Our
future studies will focus on the molecular mechanisms of epimorphin action, in
specific what are the key molecules responsible for such effects as cell-cell
adhesion, reconstitution of cytoskeleton, regulation of cell polarity and
cellular mitogenic activity. We will also try to establish technologies to
control the morphological differentiation of other types of tissues. The target
cells mainly used in the lab will include cells from skin and its epithelial
derivatives (hair follicular cells and mammary epithelial cells), either from
primary cells and differentiated stem cell derivatives.
|
|
|





