|
During
the developmental and organ regenerative processes, epithelial tissues actively
construct complex architectures, where their differentiation programs are
spatio-temporally regulated by the surrounding stroma, so that they can effectively
exert highly specialized functions in the established organs. We have focused
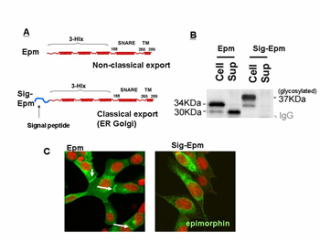
on a morphogenic protein epimorphin, which usually exists at the cytoplasmic
surface of the stromal plasma membrane and functions as a t-SNARE molecule,
while is temporally secreted extracellularly and elicits local morphogenenic
responses in the adjacent epithelia. By the last year, we determined molecular
elements for its extracellular secretion and the signaling pathway in the
target cells. This year, we tried to elucidate how its intracellular and
extracellular roles are functionally related and whether other t-SNARE
molecules (syntaxin 1, 3, 4, 5, 6) share such intriguing molecular nature. In
addition, we investigated how epimorphin signaling impacts on the cyto-differentiation
of normal keratinocytes (HaCaT). We found that 1) the domain for epimorphin's
intracellular functions (SNARE domain) could determine the direction of
epimorphin's vectorial secretion, 2) other membraneous t-SNARE molecules
(syntaxin 3, 4,) are extracellularly secreted by the similar mechanism as that
for epimorphin, and 3) the appropriate concentration gradient of epimorphin in
the epidermis is critical for the epidermal keratinization program. These
results indicate that epimorphin and its related molecules cooperatively play
regulatory roles not only on the morphological but also on the functional
differentiation in the target tissues. Recently, an important finding was
reported by researchers who have focused solely on cytoplasmic functions of
t-SNARE molecules, that is, the extracellular projection of epimorphin is
clearly detectable in the activated platelet cells, expanding its extracellular
function to hematopoietic cell types. We will further investigate the molecular
mechanisms of these intriguing "double-life" proteins to establish a
novel concept on the tissue morphoregulation. (by Hirai Y.)
 |
Another research project
currently working on is the analysis of regulatory mechanisms of tight junction
(TJ) formation in keratinocyte. We have found that the localization of ZO-1,
one of the components of TJ, changes in HaCaT, human epidermal keratinocyte
cell line, when this cell line is cultured with JNK inhibitor. In addition,
claudin-4, another components of TJ, was newly phosphorylated during this
process.
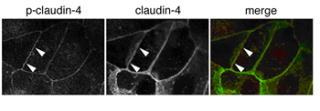
In this period, we tried to find a kinase which phosphorylates claudin-4.
During this process, we found that claudin-4 contains a sequence which could be
phosphorylatd by aPKC. Kinase assay demonstrated that the 195th serine of mouse
claudin-4 was phosphorylated by aPKC in vitro. The 194th serine of human
claudin-4 corresponding to the 195th serine of mouse claudin-4 was
phosphorylated in HaCaT cells cultured with JNK inhibitor, and the
phosphorylated claudin-4 co-localized with ZO-1 at TJ. We also found that aPKC
activity was required for both the claudin-4 phosphorylation and TJ formation
in HaCaT. These findings suggest that aPKC regulates the TJ formation through
the phosphorylation of claudin-4.
Now we are examining whether the regulatory mechanism of TJ formation found in
HaCaT cells is utilized in other cells. (by Aono S.)
 |
|





