|
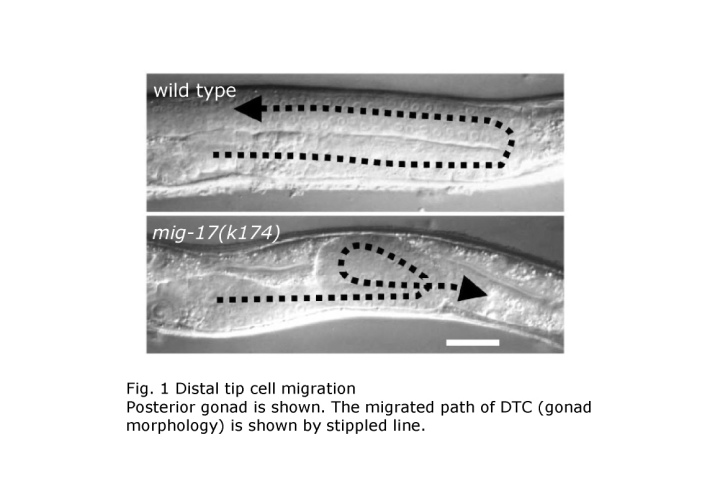
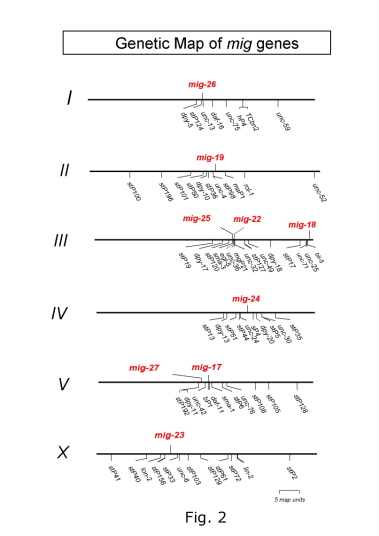
During animal development, organs are built from small rudiments surrounded by basement membranes by precise cell division, cell migration and cell shape change. The final morphology of each organ is highly variable. Cell migration is a particularly important process for shaping in organogenesis. The nematode C. elegans provides an opportunity to analyze cell migration at the cellular, genetic and molecular levels. We are especially interested in the migration of two gonadal distal tip cells (DTCs), which are located on the anterior and posterior ends of the hermaphrodite gonad primordium. The DTCs are mesodermal cells, which have their own basement membranes and migrate in a U-shaped pattern during larval development to form the anterior and posterior U-shaped gonad arms (Kimble and White, Dev. Biol. 81, 208?219, 1981; Hedgecock et al., Development 100, 365-382, 1987) (Fig. 1). C. elegans has four larval stages punctuated by molts. The migration of DTCs is coupled with the elongation of gonadal tubes and requires an appropriate interaction between the gonadal and body wall basement membranes. So far, most of the molecules known to function in distal tip cell migration have mammalian orthologs. For example, the guidance molecule UNC-6 corresponds to Netrin and the UNC-6 receptor UNC-40, which is expressed on the DTC, corresponds to DCC (Deleted in Colorectal Cancer) (Culotti and Merz, Curr. Opin. Cell Biol. 10, 609-613, 1998). Therefore, the DTC migration in C. elegans can be considered as an excellent model system to study the mechanism of organogenesis in animal development. We would like to elucidate the molecular system that operates in the morphogenesis of organs based on the C. elegans genetics and cell biology. We have identified a novel mutant class, which exhibits meandering DTC phenotypes, from isolation and analysis of DTC migration mutants (Nishiwaki, Genetics 152, 985-997, 1999) (Fig. 1). These mutants do not affect the migration itself, but rather the direction or route of migration. Nine different mig (cell migration defective) loci have been identified from the analysis of 23 isolated mutations (Fig. 2). Various double mutant combinations have revealed enhanced meandering phenotypes, sugg
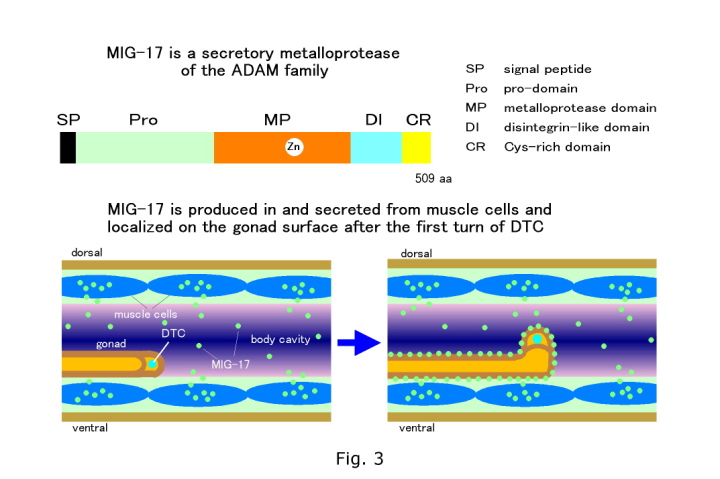
esting that they affect the same or parallel pathways. Therefore we decided to focus on this mutant class to identify more molecules, analyze molecular interactions, and clarify the novel aspects of the regulation of directional DTC migration. We have molecularly cloned one of these genes mig-17 and found that the encoded protein MIG-17 is a member of the ADAMTS (A Disintegrin And Metalloprotease with ThromboSpondin motifs) family of secreted metalloproteases (Nishiwaki et al., Science, 288, 2205-2208, 2000) (Fig. 3). The mammalian ADAMTS proteins have been implicated in physiological and pathological processes, including blood coagulation disorders, connective tissue disorders, arthritis and tumor progression (Nishiwaki et al., Science, 288, 2205-2208, 2000; Porter et al., Biochem. J., 386, 15-27, 2005). Using GFP fusion genes, MIG-17 was found to be produced in and secreted from the body wall muscle cells and subsequently localized to the surface of the gonad (probably to the gonadal basement membrane) (Fig. 3). Interestingly, MIG-17 begins to localize to the gonad after the first turn of the DTC and this timing of localization coincides with the first sign of DTC migration abnormality in mig-17 mutants. Experimental evidence supports the concept that the localization of MIG-17 to the gonadal basement membrane is important for correct migration. The MIG-17 metalloprotease appears to digest a substrate, which is not characterized yet, in the basement membrane of the gonad or the body wall so that DTCs can migrate according to directional cues. |